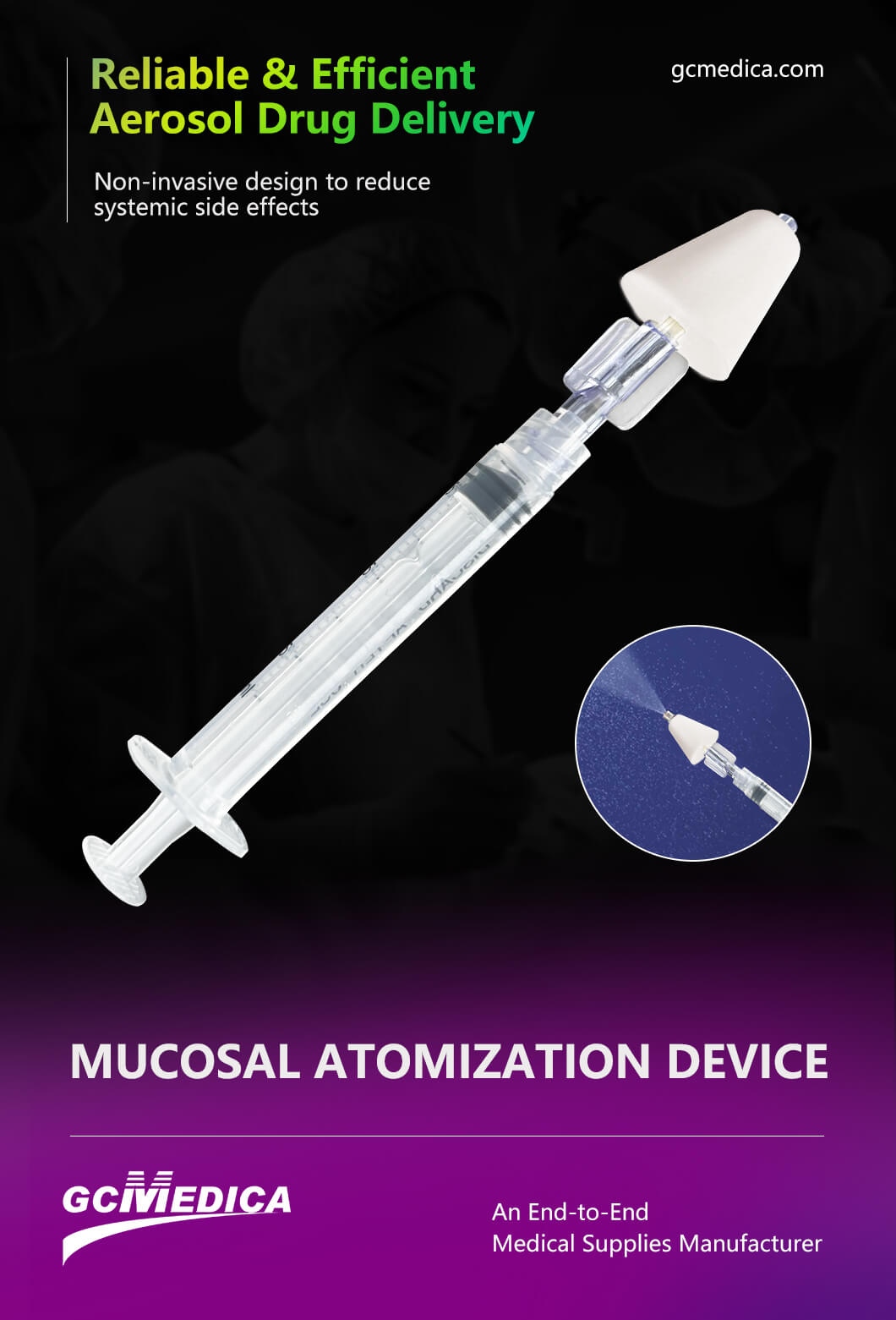
The intranasal mucosal atomization device (MAD) is a specialized tool designed to deliver medications as a fine mist directly onto the richly vascularized nasal mucosa. By converting liquid pharmaceuticals into droplets typically ranging from 30 to 100 microns in diameter, the MAD enhances rapid drug absorption and systemic bioavailability while bypassing gastrointestinal degradation and first‑pass hepatic metabolism. Originally developed for emergency use—particularly for intranasal naloxone in opioid overdose—the device has since seen broad application across pain management, sedation, seizure control, and pediatric analgesia.
Structurally, a typical MAD consists of three core components: a luer‑lock syringe, a flexible catheter or atomizer tip, and an adapter that couples the two. The syringe (commonly in 1 mL or 3 mL sizes) allows precise measurement of volume; the atomizer tip features a conical or multi‑port nozzle engineered to disperse fluid into a uniform spray; and the adapter ensures a secure, leak‑free connection. Many models incorporate color‑coded tips to denote gauge size or intended drug class, reducing the risk of administration errors in fast‑paced settings. The device is supplied in sterile, single‑use packaging to maintain infection control and simplify bedside preparation.
Clinicians deploy the MAD in diverse environments—from emergency departments and intensive care units to ambulance services and battlefield medical units. Intranasal administration is particularly advantageous when intravenous access is challenging or time‑consuming, such as in pediatric, geriatric, or dehydrated patients. For instance, intranasal fentanyl (up to 2 micrograms per kilogram) can achieve effective analgesia within 5 to 10 minutes for acute pain episodes, while intranasal midazolam (0.2 mg/kg) provides rapid seizure control without the need for IV cannulation. Studies have demonstrated comparable pharmacokinetics between intranasal and intravenous routes for many agents when delivered via a well‑designed atomizer, with peak plasma concentrations reached in under fifteen minutes for most small molecules.
Beyond emergencies, the MAD is gaining traction in outpatient and dental offices for minimal‑invasive sedation, as well as in sports medicine for on‑field analgesia. Its needle‑free design reduces patient anxiety and needle‑stick injury risk for healthcare workers. However, clinicians must account for certain limitations: excessive nasal secretions, mucosal inflammation, or anatomical obstruction (e.g., deviated septum) can impede uniform drug distribution. Optimal dosing also requires consideration of drug concentration and total volume—volumes over 1.0 to 1.5 mL per nostril may lead to runoff and diminished efficacy.
Proper technique is essential for maximizing device performance. The patient's head should be tilted slightly backward; the tip is then inserted just past the nasal vestibule, directed toward the mid‑inferior nasal turbinate at approximately a 45‑degree angle. The plunger is depressed steadily to release the atomized mist, while the patient is instructed to breathe normally and avoid sniffing or swallowing during administration. Training modules and competency checklists are widely available to ensure consistent practice.
In summary, the intranasal mucosal atomization device represents a cornerstone of modern non‑invasive drug delivery. By combining precision engineering with rapid onset, it offers an effective, patient‑friendly alternative to traditional parenteral routes—improving clinical workflows, enhancing patient comfort, and expanding therapeutic options across acute and outpatient care settings.
| Mucosal Atomization Device > |


 Français
Français Español
Español Products
Products

 About Us
About Us