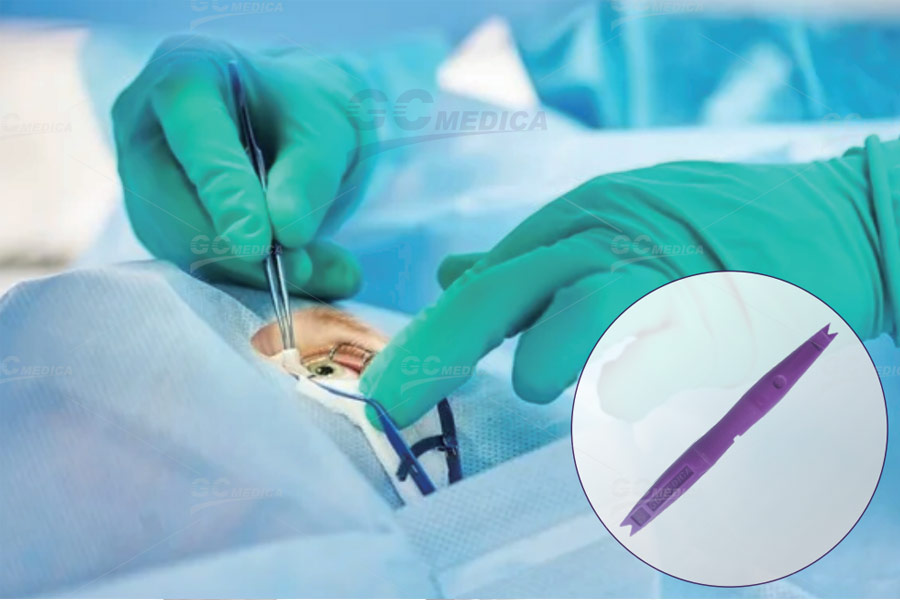
A scleral marker is an essential tool in ophthalmic diagnostics and surgery, enabling precise indentation of the globe and accurate marking of sclerotomy or injection sites. When choosing between single‑use and reusable models, practices must weigh factors such as sterility management, performance consistency, environmental impact, and workflow efficiency to ensure patient safety and procedural reliability.
Design & Material
Both single‑use and reusable scleral markers are typically crafted from surgical‑grade stainless steel for rigidity and biocompatibility. Single‑use markers arrive pre‑sterilized in sealed pouches, often featuring a flat handle with textured grip zones and dual‑ended tips (depressor and marker). Reusable models share similar ergonomics but may offer thicker shafts and reinforced tip junctions to withstand repeated autoclaving cycles. Handle profiles range from slim, round designs to wider, square cross‑sections—clinicians should select a shape that optimizes tactile feedback during indirect ophthalmoscopy.
Sterilization & Safety
Sterile integrity is non‑negotiable in ophthalmology. Single‑use scleral markers eliminate the need for reprocessing, virtually eradicating cross‑contamination risk. In contrast, reusable markers require a validated cleaning and sterilization protocol—typically involving enzymatic soak, ultrasonic cleaning, and steam sterilization—to meet regulatory standards. Practices must monitor for residual debris or corrosion, as even minute surface irregularities can harbor microbes or compromise marking precision.
Performance & Durability
Clinician experience indicates that fresh single‑use markers deliver uniformly sharp markings and smooth depression across every procedure, with no degradation in feel. Reusable markers may exhibit subtle tip wear or handle discoloration after multiple cycles; however, high‑quality stainless steel variants can endure hundreds of sterilization rounds before replacement is necessary. Regular inspection for tip deformation and handle integrity is crucial to maintain consistent performance.
Environmental & Workflow Impact
Single‑use markers generate medical waste and require proper disposal, contributing to environmental burden. Their convenience, however, streamlines operating room turnover and reduces instrument‑processing bottlenecks. Reusable models lower per‑procedure waste and long‑term material costs but depend on centralized sterile processing facilities. Practices with limited sterile processing capacity may experience scheduling delays, whereas those with robust reprocessing infrastructure can achieve faster ROI and reduced carbon footprint over time.
Selection Criteria
When deciding between single‑use and reusable scleral markers, consider:
Procedure Volume: High‑throughput clinics benefit from disposable markers to maximize turnover; lower‑volume centers may amortize reusable costs effectively.
Sterile Processing Capacity: Ensure adequate staff, equipment, and validated protocols if opting for reusable models.
Budget & Sustainability Goals: Balance upfront instrument costs against waste disposal fees and environmental priorities.
Clinical Preferences: Solicit surgeon feedback on handle ergonomics, tip sharpness, and overall instrument balance.
Conclusion
No single option suits every practice. Single‑use scleral markers offer unmatched convenience and sterility assurance, while reusable models provide long‑term cost efficiency and reduced environmental impact. By evaluating procedure demands, sterilization workflows, and sustainability targets, ophthalmic teams can select the scleral marker solution that best aligns with their clinical and operational objectives


 Français
Français Español
Español Products
Products

 About Us
About Us