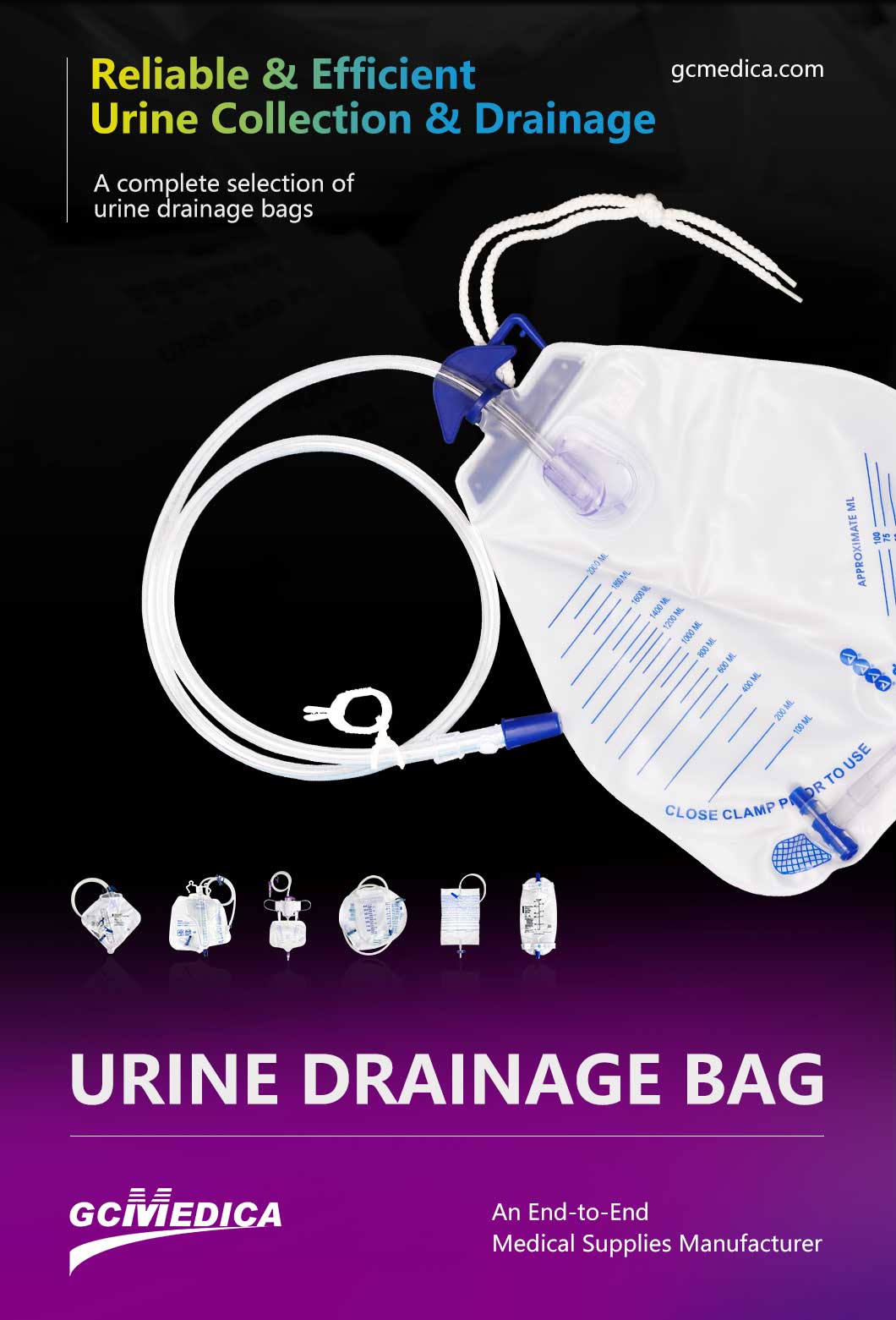
Selecting a dependable urine container manufacturer is crucial for healthcare facilities and laboratories to ensure accurate diagnostics and maintain patient safety. The quality of urine containers directly impacts specimen integrity, influencing test results and clinical decisions. This article outlines essential factors to consider when choosing a reliable supplier for urine containers.
| Learn more about urine drainage bags > |
Material Quality and Compliance
The foundation of a high-quality urine container lies in its construction materials. Opt for manufacturers that utilize medical-grade plastics or glass, ensuring durability and chemical stability. These materials should be non-reactive to prevent any interaction with urine samples that could compromise test outcomes. Additionally, verify that the materials comply with international health and safety standards, such as FDA approval, ISO certifications, or CE marking, indicating adherence to stringent quality protocols.
Design and Functionality
An effective urine container should feature a user-friendly design that facilitates easy collection, secure sealing, and leak-proof transportation. Transparent containers are advantageous, allowing for quick visual assessment of the sample's color and clarity. Graduated markings can aid in measuring urine volume accurately. The design should also prioritize patient comfort and ease of use, minimizing the risk of spillage or contamination during collection.
Sterility and Packaging
Maintaining specimen integrity requires that urine containers be sterile and individually packaged. Inquire about the manufacturer's sterilization processes and ensure they meet recognized standards. Proper packaging not only preserves sterility but also simplifies storage and distribution within healthcare settings.
Production Capacity and Lead Times
Assess the manufacturer's production capabilities to ensure they can meet your facility's demand, both in volume and delivery timelines. Consistent supply is vital to avoid disruptions in clinical operations. Discuss lead times and confirm that the supplier can accommodate urgent requests if necessary.
Quality Assurance and Certifications
A reputable manufacturer should have a robust quality management system in place. Look for certifications such as ISO 13485, which pertains to medical device quality management systems. These certifications demonstrate the manufacturer's commitment to maintaining high-quality standards throughout the production process.
Customization and Flexibility
Depending on your facility's specific needs, you may require containers of varying sizes, shapes, or with unique features. Evaluate whether the manufacturer offers customization options and is willing to collaborate on product development to meet your requirements.
Cost Considerations
While quality should never be compromised, it's essential to consider cost-effectiveness. Obtain detailed pricing information and compare it with other suppliers. Be cautious of prices that seem unusually low, as they may reflect inferior quality. Aim for a balance between cost and quality to ensure value for money.
Customer Support and Communication
Effective communication is key to a successful supplier relationship. Choose a manufacturer that offers responsive customer support, addressing inquiries and resolving issues promptly. Clear channels of communication help in coordinating orders, managing expectations, and fostering a collaborative partnership.
Reputation and References
Research the manufacturer's reputation within the industry. Seek testimonials or references from other healthcare facilities that have used their products. Positive feedback and a history of satisfied clients can provide confidence in the supplier's reliability and product quality.
Regulatory Compliance and Documentation
Ensure that the manufacturer complies with all relevant regulations and can provide necessary documentation, such as Certificates of Analysis (CoA) or Material Safety Data Sheets (MSDS). Proper documentation is essential for traceability and regulatory audits.
Conclusion
Choosing the right urine container manufacturer is a critical decision that impacts the accuracy of diagnostic tests and overall patient care. By thoroughly evaluating factors such as material quality, design, sterility, production capacity, quality assurance, customization options, cost, customer support, reputation, and regulatory compliance, healthcare facilities can establish a partnership with a reliable supplier. This strategic selection ensures the consistent availability of high-quality urine containers, supporting effective clinical operations and patient outcomes.


 Français
Français Español
Español Products
Products

 About Us
About Us