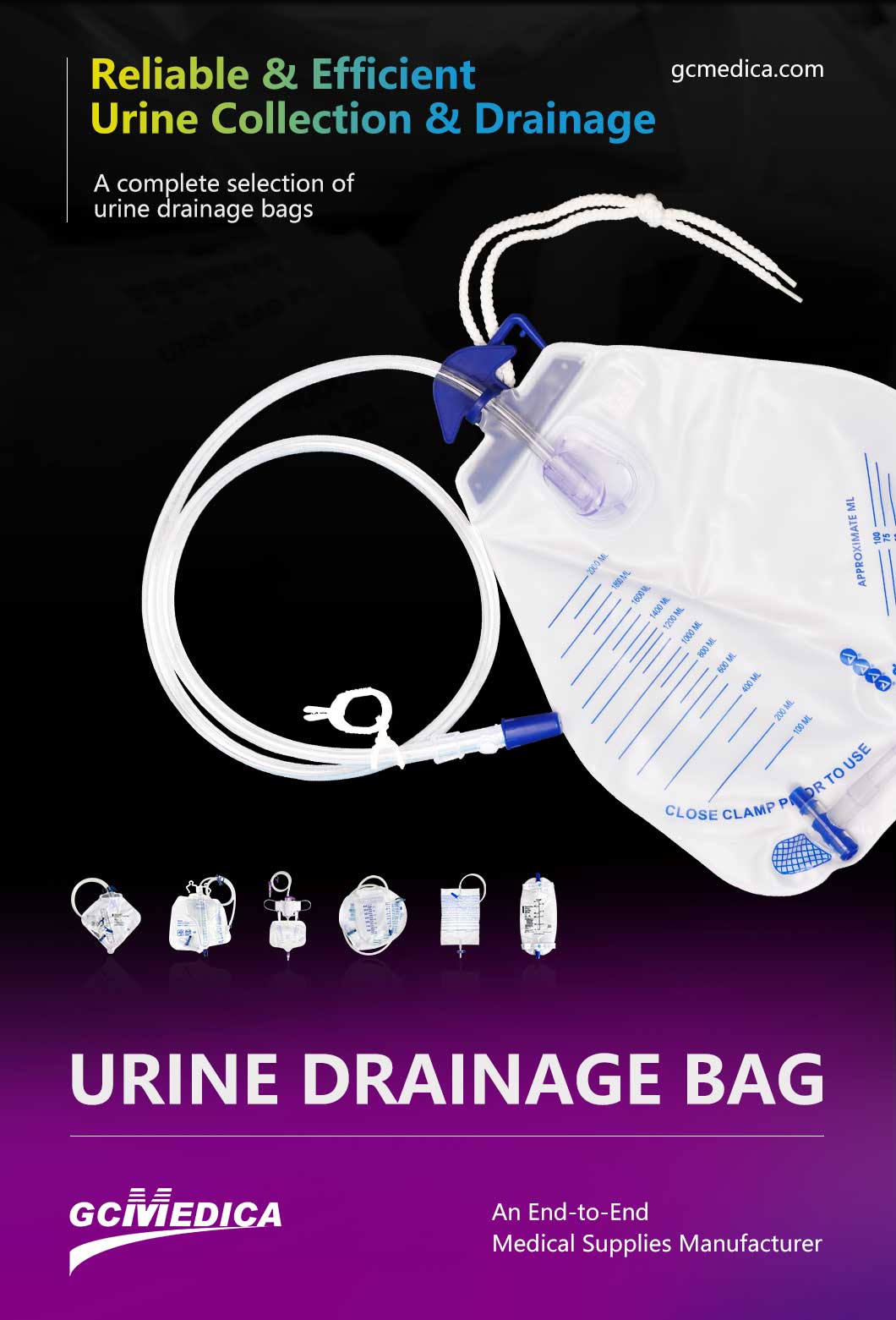
The production of urine containers is a critical aspect of medical device manufacturing, directly influencing the accuracy of diagnostic tests and patient care. Advancements in manufacturing techniques have led to the development of high-performance urine containers that meet stringent quality standards. This article explores the advanced manufacturing methods employed to produce these essential medical devices.
| Learn more about urine drainage bags > |
Material Selection and Preparation
The foundation of a high-quality urine container lies in the selection of appropriate materials. Medical-grade plastics, such as polypropylene and polyethylene, are commonly used due to their durability, chemical resistance, and biocompatibility. These materials ensure that the containers do not react with urine samples, preserving the integrity of the specimen for accurate analysis.
Injection Molding Techniques
Injection molding is a prevalent method in urine container production. This process involves injecting molten plastic into precision-engineered molds to form the desired container shape. Advanced injection molding machines offer high efficiency and consistency, producing containers with uniform wall thickness and smooth surfaces. This uniformity is crucial for ensuring leak-proof seals and reliable performance.
Automation and Assembly Lines
Modern manufacturing facilities utilize automated assembly lines to enhance production efficiency and maintain product consistency. Automated systems handle tasks such as molding, trimming, sealing, and packaging, reducing human error and increasing throughput. For instance, specialized assembly lines can produce up to 3,000 urine containers per hour, accommodating various sizes and specifications.
Sterilization Processes
Sterility is paramount for urine containers to prevent contamination of specimens. Manufacturers employ various sterilization techniques, including gamma irradiation, ethylene oxide gas, and autoclaving. These methods effectively eliminate microbial contaminants, ensuring that containers are safe for clinical use.
Quality Control and Compliance
Adherence to international standards is essential in medical device manufacturing. Urine container production aligns with standards such as ISO 6717:2021, which specifies requirements for single-use containers used in specimen collection. Compliance with these standards ensures that products meet safety and performance criteria.
Sustainable Manufacturing Practices
Environmental considerations have led manufacturers to adopt sustainable practices. The use of post-industrial recycled materials in urine container production reduces waste and conserves resources. For example, some containers are made with up to 31% recycled material, contributing to environmental sustainability without compromising product quality.
Conclusion
Advancements in urine container manufacturing techniques have significantly improved the quality and performance of these medical devices. Through meticulous material selection, precision molding, automation, stringent sterilization, and adherence to international standards, manufacturers produce containers that are reliable and safe for clinical use. Additionally, the integration of sustainable practices reflects the industry's commitment to environmental responsibility.


 Français
Français Español
Español Products
Products

 About Us
About Us