- Laparoscopic & Endoscopic Products
-
Respiratory & Anesthesia
-
Oxygen Therapy
- Capnography Mask
- CO₂ Nasal Oxygen Cannula
- Elastic Head Strap Cannula
- Oxygen Mask with Swivel Connector
- Multi-vent Mask
- Non-rebreathing Mask
- Oxygen Mask with Adjustable Nose Clip
- Venturi Mask
- Nebulizer Mask
- Nebulizer with Mouth Piece
- Nebulizer Mask with Swivel Connector
- Tracheostomy Mask
- Nasal Oxygen Cannula
-
Airway Management
- Oropharyngeal Airway
- Nasopharyngeal Airway
- Laryngeal Mask Airway
- Tracheostomy Tube
- Endotracheal Tube
- Endotracheal Tube Introducer
- Intubating Stylet
- One-way Valve Mouthpiece
- Mucus Control Vacuum Valve
- Mucus Specimen Trap
- Mucus Extractor
- Mucus Extractor with Protective Sheath
- Disposable Aspirating Tube
- Anesthesia
- Closed / Open Suction Catheter
- Pressure Infuser
- ABC Mouthpiece and Filter Kit
- MDI Spacer
- Disposable Ezscope™ Pro Broncho
- Nose Clip
-
Oxygen Therapy
- Cardiothoracic Surgery
- Gynaecology
-
Urology
- CathVantage™ Portable Hydrophilic Intermittent Catheter
-
Cysto/Bladder Irrigation Set
- M-easy Bladder Irrigation Set
- B-cylind Bladder Irrigation Set
- S-tur Bladder Irrigation Set
- S-uni Bladder Irrigation Set
- B-uro Bladder Irrigation Set
- Premi Bladder Irrigation Set
- J-pump Bladder Irrigation Set
- J-tur Bladder Irrigation Set
- H-pump Bladder Irrigation Set
- Sup-flow Bladder Irrigation Set
- Maple Irrigation Set
- Peony Irrigation Set
- Nelaton Catheter
- Urinary Drainage Bag
- Urinary Drainage Leg Bag
- Enema Kits
- Sitz Bath Kits
- Click Seal Specimen Container
- Silicone Male Catheter
- Spigot Catheter and Adaptor
- Sandalwood Irrigation Set
- Freesia Irrigation Set
- Daffodil Irrigation Set
- Enteral Feeding Products
- Dental
- Fluid Management
- Warming Unit and Warming Blanket
-
Operating Room Necessities
- Pulsed Lavage System
- Magnetic Drape
- Suction Handle
-
General Surgery
- Perfusion Atomizer System
- Gastric Sump Tube
- Surgical Hand Immobilizer
- Administration Set for Blood
- Ear/Ulcer Syringe
- Bulb Irrigation Syringe
- Toomey Irrigation Syringe
- Mixing Cannula
- Basin Liner/Basin Drape
- Camera Handle Cover
- Light Handle Cover
- Medical Brush
- Sponge Stick
- Suture Retriever
- Needle Counter
- Disposable Calibration Tube
- Heparin Cap
- 100ML Bulb Irrigation Syringe
- Scleral Marker
- Surgical Light Handle
- Mucosal Atomization Device
- Durable Medical Equipment
- Patient Handling System
- Personal Protective Equipment
- PVC-FREE Medical Device
- Emergency
-
GCMEDICA Dispositifs médicaux jetablesJul 26 , 2024
-
GCMEDICA Dispositivos médicos desechables en EuropaJul 26 , 2024
-
GCMEDICA Disposable Medical Devices In EuropeJul 26 , 2024
-
GCMEDICA Disposable Medical Devices In North AmericaJul 26 , 2024
-
GCMEDICA Dispositifs médicaux jetables en EuropeJul 26 , 2024

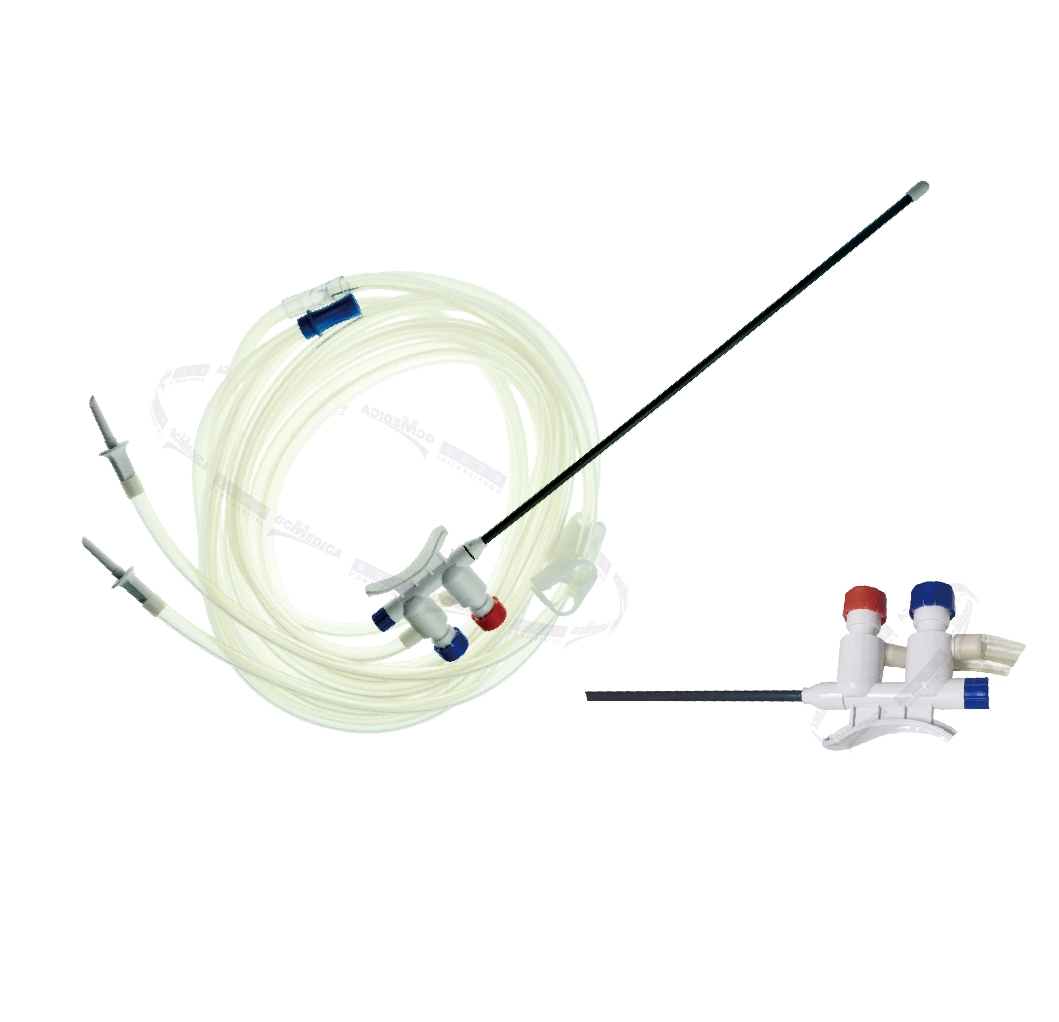
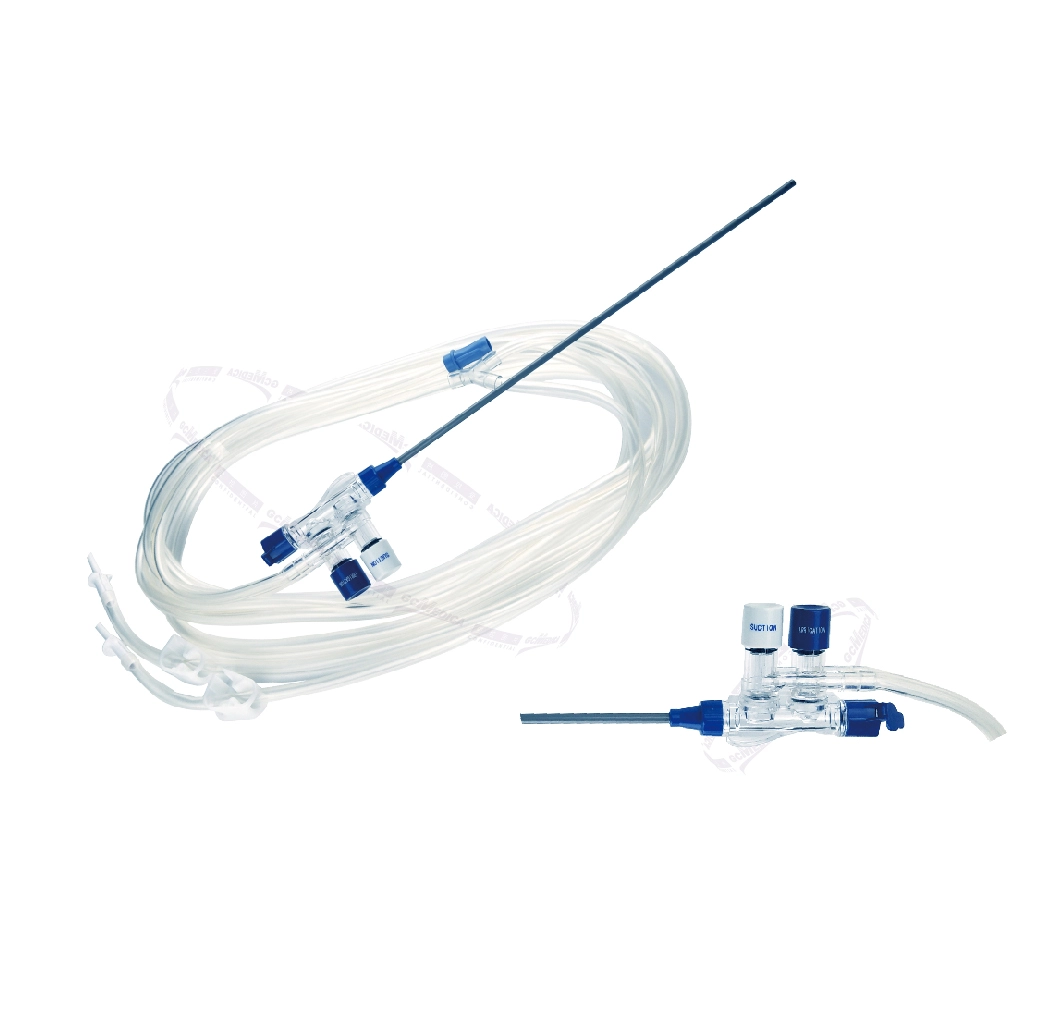
Single-use Endoscopic Valves
Single-use Endoscope Valves are critical components in the field of endoscopy, designed to maintain sterility and improve patient safety during endoscopic procedures. These disposable valves are used with endoscopes to control the flow of fluids and air, ensuring clear visibility and operational efficiency for the physician. Their single-use nature is a direct response to concerns about cross-contamination and the challenges of effectively cleaning and sterilizing reusable valves.
Sterile single-use endoscopic valves help prevent potential patient safety risks and eliminate the need for manual cleaning and reprocessing.
Packaged sterile. Valves easily incorporate into your infection prevention policy as a single-patient use item.
Features of Single-Use Endoscopic Valves
Sterile Single Use Air/Water, Suction, and Biopsy Valves:
Eliminate manual cleaning and reprocessing of reusable valves
Help create consistent practices
Reduce the potential for errors
Available for Olympus®, Fuji®, and Pentax® Endoscopes
Specification of Single-Use Endoscopic Valves
| No. | Product code | Description |
| 01 | GC1965O01 | 4-piece Kit (Single Use Air/Water, Suction and Biopsy Valves and Connector ) |
| 02 | GC1965O02 | 3-piece Kit (Single Use Air/Water, Suction and Biopsy Valves) |
| 03 | GC1965O03 | 2-piece Kit (Single Use Air/Water and Suction Valves) |
| 04 | GC1965O04 | Biopsy Valves |
Regulatory and Quality Standards
Single-use endoscopic valves must comply with rigorous regulatory standards to ensure their safety and efficacy. In the United States, for example, they would be subject to FDA oversight, requiring premarket notification [510(k)] or premarket approval (PMA), depending on their classification. Similarly, in Europe, they must comply with the Medical Devices Regulation (MDR) and carry the CE mark. Manufacturers must demonstrate that these products meet strict quality and performance criteria, including biocompatibility, sterility, and safe disposal.
Frequently Asked Questions (FAQs)
Q1:What Are the Benefits of Single-use Endoscopic Valves Compared to Reusable Valves?
A1:Single-use endoscopic valves offer significant benefits over their reusable counterparts, primarily in enhancing patient safety and procedural efficiency. They eliminate the risk of cross-contamination between patients, addressing a critical concern in infection control. Without the need for time-consuming and sometimes imperfect cleaning and sterilization processes required for reusable valves, single-use valves ensure a high level of sterility and compliance with stringent health regulations. Additionally, they provide consistent performance and eliminate the possibility of wear and tear associated with repeated use, which can compromise patient safety and the effectiveness of procedures.
Customer Reviews for GCmedica Endoscopic Valves
1.Hiroshi Tanaka
★ ★ ★ ★ ★"Our clinic has seen a reduction in HAIs since we adopted single-use endoscopic valves. This has not only improved patient outcomes but also enhanced our reputation for providing safe, high-quality care."